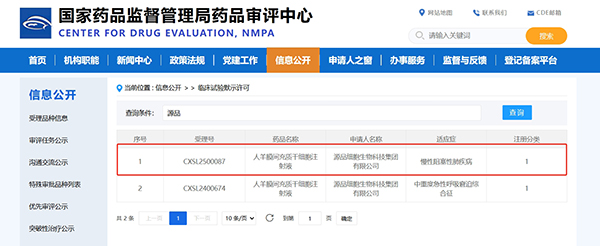
Source Bio receives another stem cell IND approval, targeting chronic lung treatment for over 100 million patients!
On April 24, 2025, the State Drug Administration (SDA) formally approved the clinical trial (IND) of Human Amniotic Membrane Mesenchymal Stem Cell Injection (HAMSC Injection), a Class 1 biologic product independently developed by Genpine Biologics, for the indication of Chronic Obstructive Pulmonary Disease (COPD). COPD.) This is another milestone breakthrough in the treatment of respiratory diseases, following the clinical approval of the product in the field of moderate-to-severe acute respiratory distress syndrome, which will ignite a new hope for more than 100 million COPD patients in China.
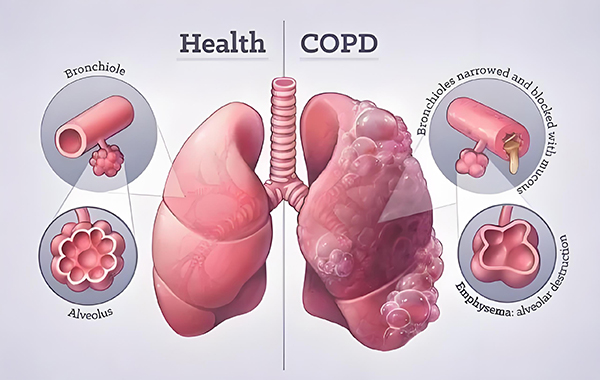
Chronic Obstructive Pulmonary Disease (COPD) is a highly prevalent chronic disease with more than 100 million patients in China, and is the third leading cause of death in China. It is characterized by symptoms such as dyspnea, cough and sputum, and is mainly due to persistent airflow obstruction caused by abnormalities in the airways and/or alveoli. During the long course of the disease, it is prone to acute exacerbations, with a narrow treatment window, and patients are often accompanied by interstitial lung abnormalities and even pulmonary fibrosis, which can easily lead to respiratory failure to the point of death. Currently, there is no effective cure for chronic obstructive pulmonary disease (COPD), which can only be alleviated and controlled by inhalation medication over a long period of time. Once it develops into pulmonary fibrosis, existing treatments cannot reverse the progressive decline in respiratory function, resulting in a shorter life expectancy and increased risk of death.
“As a Class 1 therapeutic biological product that has not yet been marketed at home or abroad, Human Amniotic Membrane Mesenchymal Stem Cell Injection is the world's only innovative drug that uses human amniotic membrane tissue as the source of mesenchymal stem cells. Compared with umbilical cord, chorionic villus and adipose-derived MSCs, amniotic membrane MSCs express a higher level of genes related to inflammation regulation and epithelial differentiation, and have a good potential to inhibit inflammation and promote the repair of bronchial epithelial tissue or lung epithelial tissue. The results of cytokine microarray studies also show that amniotic MSCs can secrete relatively higher levels of cytokine combinations related to tissue repair and inflammation regulation, suggesting that they are more advantageous in the treatment of COPD.
The research team of Genpine Bio has always been focusing on “patient benefit”, responding to the call of policy, promoting the clinical translation and application of cellular products with high quality, and committing to transforming more cellular application technologies into reliable clinical therapeutic products. The company has established a leading pilot platform for cell drug R&D and production, and has improved the whole chain R&D system of basic research, pharmacological research, non-clinical research, clinical research and registration declaration for cell application. In the future, Source Bio will continue to promote the clinical transformation of cell therapy technology, fulfill the mission of “all for the sake of human life and health”, and provide safer and more efficient breakthrough therapies for patients worldwide.






