After the President focused on stem cells, the provinces set off what policy bonus storm?
On May 2, 2018, Xi Jinping, General Secretary of the CPC Central Committee, President of the People's Republic of China and Chairman of the Central Military Commission, came to Peking University for an inspection tour, and Professor Deng Hongkui of the School of Life Sciences of Peking University, with the topic of “new generation of stem cell technology and its application”, showed the scientific research achievements of the team to President Xi Jinping and exchanged views with the General Secretary eagerly.
The General Secretary highly affirmed China's new-generation stem cell research achievements and said: “I am proud of you after seeing your achievements. Innovation is the first driving force leading the development, and is the most crucial factor of the country's comprehensive national strength and core competitiveness. Major scientific and technological innovations are the weight of the country, the country's weapon, must be firmly in their own hands, must rely on self-reliance, independent innovation.

Xi Jinping visits Peking University's “New Generation of Stem Cell Technology and Its Applications” Achievement Exhibition
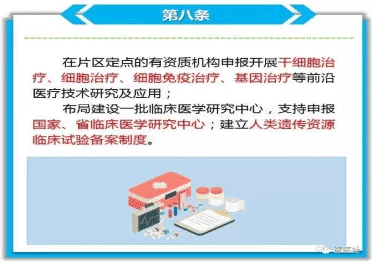
Since General Secretary Xi Jinping's speech on May 2, 2018, the State Council has introduced relevant policies to support clinical cutting-edge medical technology research in cell therapy; the central financial allocation, the State Drug Administration, the Ministry of Science and Technology has introduced a series of support initiatives; Shandong, Guangdong, Anhui, Shanghai, Beijing, Tianjin, Zhejiang, Yunnan, Sichuan, Chongqing and other provinces and municipalities directly under the central government have issued programs to support the translational research of stem cells, provinces and municipalities directly under the central government have successively issued plans, programs and opinions to support stem cell translational research, and listed stem cell technology and translational research as deepening development and key support projects to promote the development of stem cell industry.
01 General Secretary Xi Jinping makes important original breakthroughs using stem cell technology
On May 28, 2018, General Secretary Xi Jinping attended the opening ceremony of the 19th Academician's Conference of the Chinese Academy of Sciences and the 14th Academician's Conference of the Chinese Academy of Engineering and delivered an important speech - We are focusing our efforts on advancing basic and applied basic research, and we will make important original breakthroughs using stem cell technology.
02 State Drug Administration Accepts Application for Clinical Registration of Stem Cell Therapy
On June 8, 2018, the State Drug Administration (SDA) newly accepted the clinical registration application for stem cell therapy, heralding China's relaunch of the clinical application of stem cell therapy. According to statistics, since the launch of the first batch of pilot special projects of the National Key Research and Development Program in 2015, this key special project has been supported by the central financial allocation for three consecutive years so far: in 2016, 25 projects, 480 million; in 2017, 43 projects, 940 million; in 2018, 30 projects, 580 million, totaling more than 2 billion yuan. This fully reflects the high importance that the state attaches to the field of stem cells and regenerative medicine from the national top-level design of the science and technology innovation system.
03 State Ministry of Science and Technology: support for stem cell and translational research project declaration
On August 21, 2018, the Ministry of Science and Technology of the People's Republic of China (MOST) officially announced the “Stem Cells and Translational Research” Pilot Project Declaration Guidelines for 2019 (Draft for Comments)”, which explicitly proposed that in 2019, China will conduct clinical research on cell therapy using clinical-grade stem cell products for a certain kind of major diseases or injuries in the neurological, respiratory and digestive systems. 2019 is the first year that clinical research on cell therapy will be conducted. On January 21, 2019, the Ministry of Science and Technology (MOST) released the declaration guidelines for six key special projects under the National Key Research and Development Program (NKRDP), including “Stem Cell and Translational Research”. Among them, the medicine and health-related special projects include “stem cell and translational research”, “nanotechnology” and “protein machine and life process regulation”.

04 State Council: Supporting Research Programs on Clinical Frontier Medical Technologies of Stem Cells
On October 16, 2018, the Chinese government website released the “Approval of the State Council on Agreeing to Establish the China (Hainan) Pilot Free Trade Zone”, in which the State Council agreed to establish the China (Hainan) Pilot Free Trade Zone. The program clearly puts forward: relying on Boao Lesheng International Medical Tourism Pilot Zone, vigorously develop international medical tourism and high-end medical services, the scope of the Pilot Zone really need to be imported, the tariff rate of the higher part of the medical equipment research appropriate reduction of tariffs. Support the development of stem cell clinical cutting-edge medical technology research projects. Relying on the existing foundation of the pharmaceutical industry, exploring the development of a pilot project for the transfer and transformation of the results of the major national scientific and technological special project for the creation of major new drugs
On November 23, 2018, the State Council issued the Notice on Several Measures on Supporting the Deepening of Reform and Innovation in the Pilot Free Trade Zone, which made it clear that medical institutions in the Pilot Free Trade Zone can carry out research projects on clinical frontier medical technology of stem cells in accordance with their own technological capabilities and in accordance with relevant regulations.

The implementation of this measure once again demonstrates the importance of China's government authorities for the development of stem cell therapy technology, and also signifies that China's stem cell industry has entered a period of rapid development under the support of national policies, and that stem cell technology will make an important contribution to the advancement of the construction of a healthy China and the improvement of people's health.
05 Ministry of Science and Technology, Ministry of Finance: approved the construction of two national stem cell banks
On June 5, 2019, the Ministry of Science and Technology (MOST) and the Ministry of Finance (MOF) jointly released the Notice on the Optimization and Adjustment List of National Science and Technology Resource Sharing Service Platforms, in which the national authorities approved the construction of two more state-level stem cell banks, namely the National Stem Cell Resource Bank, which is constructed relying on the Institute of Zoology of the Chinese Academy of Sciences (CAS), and the National Translational Stem Cell Resource Bank, which is constructed relying on Tongji University.

01 Shandong Provincial Government Accelerates Bed Application of Stem Cells and Regenerative Medicine and Other Technologies Pro
On July 2, 2018, the People's Government of Shandong Province officially released the “Shandong Provincial Medical and Health Industry Development Plan (2018-2022)”, which explicitly proposes to accelerate the clinical application of cutting-edge technologies such as stem cells and regenerative medicine, biotherapy, gene detection and targeted therapy, and to enhance the scientific and technological support capacity for the prevention and treatment of major diseases. With preventive medical services as the core, accelerate the improvement of preventive healthcare industry chain, and promote the transformation of disease treatment to health promotion. Strengthen preventive medical scientific research, accelerate breakthroughs in key technologies such as biomedical testing, biotherapy and cell therapy, develop early screening and diagnosis based on genetic testing, and support the development of personalized prevention and treatment services. Vigorously develop biotechnology drugs. Focusing on innovative frontiers and key technologies such as genetic engineering and new vaccines, accelerate the research and development of new biotechnology drugs such as stem cell technologies and products.
In December 2018, the Development and Reform Commission of the Jinan Municipal People's Government officially released on its official website the notice of the “Jinan City Ten Hundred Billion Industry Investment Guide Directory” and “Jinan City District and County Investment Industry Layout Opinions of”, which explicitly includes the cell therapy-related technologies, cell storage, cell preparation, cell inspection, cell technology application research; cell transplantation regeneration and repair of skin technology, human tissue Regeneration, cellular beauty technology application research; gene technology, gene drugs, gene therapy drugs as a 100 billion key investment projects.


02 Guangdong Provincial Government Supports Social Force Cooperation in Stem Cell Clinical Research
On July 2, 2018, the Guangzhou Municipal Government released the “Guangzhou Municipal Support for Social Forces to Provide Multi-level and Diversified Medical Services to Promote the Implementation Plan for the Accelerated Development of Social-run Medical Services”.

The program clearly puts forward that social-run medical institutions with strength are encouraged to aim at the forefront of medicine, form advantageous discipline teams, and provide medical services featuring advanced medical technologies. Promote the development of precision medicine, personalized medicine and other services in a steady and orderly manner. Promote the standardized and regulated application of new individualized biological therapy products approved in accordance with the law. Support qualified tertiary hospitals to cooperate with qualified social forces to declare and carry out clinical research on stem cells.
03 Anhui Provincial Government: accelerating cell therapy technology innovation and clinical application
On July 18, 2018, the Anhui Provincial Government issued the Notice of the Anhui Provincial People's Government on Issuing Several Policies to Support the Development of Modern Medical and Pharmaceutical Industries (Anhui Government [2018] No. 58), in which the policy explicitly states that it supports the construction of basic capacity for the development of major medical and pharmaceutical innovations such as stem cell banks and translational medicine research centers, and that the projects identified by the evaluation will be subsidized in accordance with 10% of the investment in key equipment To be subsidized, the maximum 20 million yuan. In addition, to support medical technology innovation and transformation and application, to overcome difficult and complicated diseases. Accelerate the promotion of tumor immunotherapy, cell therapy, regenerative medicine and other modern medical technology innovation and clinical application, the evaluation of the project identified as having the conditions to undertake, in accordance with the scientific research and application of the promotion of 30% of the costs incurred to be funded, up to 5 million yuan.
04 Shanghai Municipal Government: to create immune cell and stem cell therapy industry clusters
On July 25, 2018, the Shanghai Municipal People's Government released Several Opinions on Promoting the High-Quality Development of the City's Health Service Industry and Accelerating the Construction of a First-Class Medical Center City, which promotes the standardization and normalization of the application of new individualized biotherapeutic products, and creates an industrial cluster for immune cell therapy, stem cell therapy, and genetic testing. Encourage enterprises to cooperate with medical institutions and scientific research institutions to promote joint research on diagnostic and treatment technologies for major diseases. Encourage powerful medical institutions to aim at the medical frontier, vigorously promote the construction of clinical application transformation mechanisms for ethical, mature and reliable cutting-edge medical technologies, and set up advantageous discipline teams to provide advanced medical technology services. Promote the construction of precision medicine industry public service platforms such as municipal precision medicine research institutes, biological sample banks, gene banks, and the National Engineering Laboratory of Medical Big Data Application Technology, establish incubation bases for precision medicine scientific and technological achievements, and cultivate a number of international first-class precision medicine service enterprises. Develop cell therapy, gene therapy and liquid biopsy technology, promote the standardization and normalization of the application of new individualized biological therapy products, and create industrial clusters of immune cell therapy, stem cell therapy and genetic testing. Promote the aggregation and development of third-party medical testing enterprises. Encourage enterprises to cooperate with medical institutions and scientific research institutions, promote joint research on precision diagnosis and treatment technologies for major diseases, especially malignant tumors, establish precision medical demonstration bases for liver cancer, lung cancer, breast cancer, etc., and actively promote precision diagnostic and treatment programs. Optimize the management of clinical application of new medical technologies and accelerate the construction of a clinical quality control system for precision medical services. Support medical institutions to carry out genetic testing services, and incorporate genetic testing programs with precise clinical needs and high cost-effectiveness into the medical insurance payment catalog.
05 Guangdong Provincial Department of Science and Technology: supporting the declaration of scientific research projects in the field of precision medicine and stem cells
On September 15, 2018, in order to comprehensively implement the spirit of the 19th CPC National Congress and the series of speeches of General Secretary Xi Jinping on strengthening the key core technologies, in accordance with the 12th Party Congress of Guangdong Province, the 4th Plenary Session of the 12th Session, and the relevant deployment of the province's Science and Technology Innovation Conference, and in accordance with the “Implementation Plan of Guangdong Province's Key Areas of Research and Development Programs,” the Provincial Key Areas of Research and Development Programs for the Years of 2018-2019 are hereby initiated. The “Precision Medicine and Stem Cells” major scientific and technological special project declaration, this special program aims to make a major breakthrough in the core technology and technical standards of stem cell production and preparation, stem cell transplantation and the therapeutic effect and mechanism of the relevant diseases, etc., and to develop stem cell technology and products with independent intellectual property rights and verify their safety and efficacy through the corresponding clinical research, and to form the corresponding clinical research. The project aims to develop stem cell technologies and products with independent intellectual property rights and verify their safety and effectiveness through corresponding clinical research, form corresponding technical standards, cultivate and drive emerging industries, and promote the overall level of regenerative medicine research and industrialization in the province to significantly improve, and some key technologies and products to reach the international leading level.
06 Shanghai Municipal Health Planning Commission: creating a collaborative innovation cluster for stem cell therapy
On September 17, 2018, the Shanghai Municipal Health Planning Commission issued a Notice on the Call for Collaborative Innovation Clusters, which explicitly proposed that in order to implement the “Outline of the National Medical and Health Care Service System Plan (2015-2020)”, the “13th Five-Year Plan” for Special Planning of Health and Health Science and Technology Innovation, and the “Development of Shanghai's Medical Science and Technology Innovation The “13th Five-Year Plan” requires the decision to create a collaborative innovation cluster, which will be designed around the stem cell therapy of major diseases, applied basic research, preclinical research, clinical and translational medicine research integration.
07 Guangdong Provincial Government: focus on supporting the innovation and development of stem cell industry
The official website of the Guangzhou Municipal People's Government officially released the “Guangzhou Biomedical Industry Innovation and Development Action Plan (2018-2020)” on September 27, 2018, which makes it clear that the plan: focuses on supporting the innovative development of the stem cell and regenerative medicine industry, supports the development of regional cell preparation centers and cell storage centers on a pilot basis, promotes the standardization of research on stem cell products, and treats cardiovascular system diseases with stem cells, diabetes and neurological diseases as a breakthrough, and support the research and development of new therapeutic stem cell technologies and products in the application of major intractable diseases.
At the same time, it will focus on strengthening the applied basic research and translational research on stem cells, and strengthening the standardized clinical application of new therapeutic means such as cell therapy. Focus on the development of stem cell-assisted therapy for malignant tumors, regenerative repair therapy for major diseases such as central nervous system injury, skin injury and other tissue injury, digestive system diseases, etc., and research and development of new therapeutic stem cell technologies and products.
08 Beijing Municipal Government: focus on supporting basic research on stem cells and regenerative medicine
On September 27, 2018, in order to deeply implement the decision-making deployment of the State Council on promoting the healthy development of the pharmaceutical industry, conscientiously implement the “Guiding Opinions of Beijing Municipality on Accelerating Scientific and Technological Innovation for the Development of Pharmaceutical and Healthcare Industry”, further promote the high-quality development of the pharmaceutical and healthcare industry in the city, and accelerate the construction of the industry-university-research-medicine collaborative innovation system, the Beijing Municipal Plan of Action for Accelerating Collaborative Innovation in Pharmaceutical and Healthcare is formulated (2018 The Plan of Action for Accelerating Collaborative Innovation in Medicine and Health in Beijing (2018-2020) is hereby formulated. Increase support in key areas. In some cutting-edge fields, new R&D institutions will be built, while a number of large scientific devices will be laid out and constructed to support the cultivation of original innovations and accelerate breakthroughs in cutting-edge technologies. Formulate a directory of key directions for collaborative innovation development in medicine and health in Beijing, focusing on supporting basic research in stem cells and regenerative medicine, brain science and brain-like, structural biology, synthetic biology, proteomics and other basic researches, promoting the development of technologies such as immunotherapy, gene detection and new sequencing, multi-modal cross-scale biomedical imaging, and facilitating the development of innovative medicines and high-end medical devices, as well as the development of new business models in the integration of medicine and health with AI and big data technologies. and artificial intelligence, big data technology, and the integration of new business forms.

09 Tianjin Municipal Government: carrying out the construction of stem cell storage center
On November 7, 2018, the Tianjin Municipal People's Government officially released on its official website the Notice of the Tianjin Municipal People's Government General Office on the Issuance of the Three-Year Action Plan for the Development of the Biomedical Industry in Tianjin (2018-2020), which explicitly proposes: to carry out clinical research on stem cells and cellular immunotherapy drugs for hematological disorders, and to accelerate the development of stem cell drugs and regenerative medicine , gene therapy, immune cell therapy, chimeric antigen receptor T-cell immunotherapy (CAR-T cell therapy), precision medicine, high-end medical treatment and other development and application. Encourage the further opening of medical biological resources collection and storage, carry out the construction of stem cell storage centers, and accelerate the development of the cell industry.

On September 30, 2019, the People's Government of Binhai New Area, Tianjin officially issued the Action Plan for Cell Industry Technology Innovation in Binhai New Area, with the goal of building Binhai New Area into a cell therapy demonstration area. It has built a clinical trial and application base for new biomedical technologies represented by cell and gene therapy, and created a healthcare demonstration area that radiates across the country. In addition, the Binhai New Area will be built into a policy innovation pioneer area. It will innovate the policy and regulatory system, explore the graded and classified management of cell therapy, and promote the safe, standardized and high-quality development of the cell industry. Meanwhile, in terms of policy innovation, it will strive for national pilot policies.
10 Beijing Municipal Health Commission: promoting the standardization and orderly development of stem cell clinical research
On November 22, 2018, the Beijing Municipal Commission of Health issued the Guidelines on In-hospital Quality Management of Stem Cell Preparations for Stem Cell Clinical Research in Collaboration with Medical Institutions, which applies to the quality management of stem cell preparations by medical institutions in accordance with the Measures for the Management of Clinical Research on Stem Cells (for Trial Implementation), when medical institutions collaborate with preparation institutions and are supplied by them with stem cell preparations to carry out clinical research, in order to promote stem cell clinical research in a standardized and orderly manner.
11 Zhejiang Provincial Government: providing stem cell and regenerative medicine medical services
On November 28, 2018, the People's Government of Zhejiang Province issued the Implementing Opinions on Supporting Social Forces to Provide Multi-level and Diversified Medical Services, proposing to support social forces to provide cutting-edge medical services such as third-party medical testing, stem cell and regenerative medicine, immunity and gene therapy, and to promote the development of precision medicine, personalized medicine and other services in a steady and orderly manner.
12 Shanghai Municipal Government: promoting the high-quality development of stem cells and regenerative medicine
On December 5, 2018, the General Office of the Shanghai Municipal People's Government issued the Action Plan for Promoting the High-Quality Development of Shanghai's Biomedical Industry (2018-2020), which provides full support for the development and growth of various types of scientific research institutions in Shanghai and focuses on the hot directions such as stem cells and regenerative medicine. Adhere to the enterprise main body, market-oriented, and actively create a new pattern of industrial development with the combination of industry, academia, research and medicine, upstream, midstream and downstream articulation, and synergy between large, medium and small enterprises.
13 Guangdong Provincial Government: approved to carry out stem cell clinical medical technology research
On February 22, 2019, the People's Government of Guangdong Province issued the Circular on the Division of Work Program for Supporting Certain Measures to Deepen Reform and Innovation in the Pilot Free Trade Zone (PFTZ), approving that medical institutions in the PFTZ may carry out research projects on clinical cutting-edge medical technology of stem cells in accordance with their own technological capabilities and in accordance with relevant regulations.
14 Shanghai Municipal Government: supporting the construction of stem cell production and research platforms
On March 22, 2019, the Shanghai Municipal People's Government issued a notice on Several Measures to Support the Deepening of Reform and Innovation in the Pilot Free Trade Zone, which supports the construction of a stem cell production center, a stem cell quality inspection service platform, and a national stem cell resource bank and a national stem cell clinical research function platform in the Shanghai Pilot Free Trade Zone, as well as the improvement of the protection mechanism for stem cell researchers and subjects.

On December 10, 2019, nine departments, including the Shanghai Municipal Health Commission, the Shanghai Municipal Drug Administration, the Shanghai Municipal Development and Reform Commission, the Shanghai Municipal Science and Technology Commission, the Shanghai Municipal Commission of Economy and Informatization, the Shanghai Municipal Bureau of Human Resources and Social Security, the Shanghai Municipal Bureau of Finance, the Shanghai Municipal Bureau of Healthcare Security, and the Shanghai Municipal Administration of Traditional Chinese Medicine, jointly issued a Implementation Program on Strengthening Clinical Research in Medical and Healthcare Institutions to Support the Development of Biomedical Industry”. The document clearly puts forward: to promote the clinical application of biotherapeutic technologies and major innovative products. Promote and optimize the management of clinical application of new individualized biotherapeutic technologies; strive for the pilot clinical application of advanced biotherapeutic technologies such as stem cells and immune cell therapy; and support the standardization of new individualized biotherapeutic products and standardized application.
15 Chongqing Municipal Government: listing the biomedical industry as a pillar industry key development direction
On May 10, 2019, the Chongqing Municipal People's Government issued the Notice of Chongqing Municipal People's Government on the Issuance of Chongqing Municipal Special Action Program for Promoting High-Quality Development of the Manufacturing Industry (2019-2022), which proposes to list the biopharmaceutical industry as a key development direction of the pillar industry, and to actively explore the layout of the CAR- based T (chimeric antigen receptor T-cell immunotherapy) and other genetically recombinant T-cell therapeutic drugs and cellular biologics such as mesenchymal stem cells, neural stem cells, hematopoietic stem cells, and so on.
16 Zhejiang Provincial Science and Technology Department: implement cell therapy technology innovation and development pilot
On May 27, 2019, Zhejiang Provincial Department of Science and Technology released the Implementation Opinions on Accelerating the Development of Life and Health Science and Technology Innovation (Draft for Opinion), emphasizing the early and pilot implementation of cell therapy technology innovation and development pilots in the International Medical Tourism Pilot Zone, the Free Trade Zone, and the regions with a good foundation for development in a scientific and steady manner. Meanwhile, it will strengthen the prospective research in cell and immunotherapy and other fields.
17 Chengdu Municipal Government: vigorously develop stem cell and regenerative medicine technology
On July 1, 2019, the Chengdu Municipal People's Government issued the Implementation Opinions on Promoting the High-Quality Development of Chengdu's Pharmaceutical and Health Industry, vigorously developing cutting-edge technologies such as stem cell and regenerative medicine and gene therapy, and focusing on the development of innovative drugs such as novel antibodies, immunotherapy, stem cell therapy, anti-aging drugs and tumor vaccines.
18 Shenzhen Municipal Government: support the development of stem cell clinical medical technology research
On July 2, 2019, the Shenzhen Municipal People's Government issued the Circular on the Work Program of Several Measures to Support Deepening Reform and Innovation in the Pilot Free Trade Zone, stating that medical institutions in the Pilot Free Trade Zone may carry out research projects on clinical cutting-edge medical technology of stem cells in accordance with their own technological capabilities and in accordance with relevant regulations.

On January 20, 2020, the official website of Shenzhen Development and Reform Commission published two supporting documents, namely the “Guidelines for Promoting the Clustering and Development of the Biomedical Industry in Shenzhen” and the “Implementation Plan for the Clustering and Development of the Biomedical Industry in Shenzhen (2020-2025)” and the “Action Plan for the Development of the Biomedical Industry in Shenzhen (2020-2025)”, and at the same time, announced the Measures to Promote the Clustering Development of Biomedical Industry in Shenzhen”. Four documents were issued at the same time, which can be seen on the biomedical industry to support the strength of. The cell field focuses on supporting stem cell therapy, cellular immunotherapy, etc., and promoting the application of advanced technological innovations such as stem cells in Shenzhen!
19 Hebei Province: cell therapy, early and pilot test!
In November 2019, the Hebei Provincial Government Information Office held a press conference to explain “a number of policies to support the high-quality development of the biomedical industry”. For the approval, registration, supervision and other matters in the field of medicine, the reform requirements of simplifying the links and optimizing the process are put forward; for stem cells and regenerative medicine, gene therapy, tumor immunity and other new technologies and new forms of business, it is encouraged to adopt the early and pilot test, inclusive and prudent regulatory approach.
On November 25, 2019, the General Office of the People's Government of Hebei Province issued a notice on “Several Policies on Supporting the High-Quality Development of Biomedical Industry”. The notice makes it clear that the pilot study of stem cell application will be carried out. Support the Beidaihe National Life and Health Industry Innovation Demonstration Zone and the Shijiazhuang National High-tech Industrial Development Zone to carry out the construction of cell preparation centers, cell storage centers and clinical research pilots, encourage the further opening of the collection and storage of medical biological resources, carry out the third-party cell quality inspection and testing services, promote the standardization of stem cell products, and develop a standardized process, large-scale production, centralized supply, and unified Promote the standardization of stem cell products and develop a new stem cell industry development model with standardized processes, large-scale production, centralized supply and unified supervision;
Encourage Beidaihe National Life and Health Industry Innovation Demonstration Zone and Shijiazhuang National High-tech Industrial Development Zone to set up expert advisory committees to carry out stem cell health and beauty and other related businesses under their guidance in accordance with the law and regulations; and support the research on clinical diagnosis and treatment technologies of stem cell therapy and regenerative medicine, gene therapy and tumor immunity.
Encourage qualified clinical hospitals in the province to cooperate with biomedical enterprises to build a provincial technological innovation platform for individualized cell therapy. In accordance with the law and regulations to carry out a leading level of stem cell therapy, gene therapy, regenerative medicine, tumor immunity clinical diagnosis and treatment of new technologies, new methods of research and demonstration of application, merit included in the provincial science and technology programs, to give a maximum of 1 million yuan of provincial science and technology special funding support;
Support China (Hebei) Pilot Free Trade Zone biomedicine and life health industry open development. Support qualified medical and health institutions in the Pilot Free Trade Zone to carry out stem cell clinical frontier medical technology research projects in accordance with relevant regulations, and establish a green channel for project filing;
Promoting the development of life science and biotechnology innovation in Xiongan New Area. Encourage enterprises to conduct research on new technologies such as immune cell therapy, monoclonal antibody drugs, gene therapy, tissue engineering, etc., and allow them to carry out new biological therapy business in accordance with the law. Establish a filing system for clinical trials on human genetic resources. Support the construction of a genetic data center in Xiongan Area.
On January 3, 2020, Shijiazhuang Municipal Party Committee and Municipal Government issued “Several Opinions on Supporting the High-Level, Open and High-Quality Construction of Zhengding Area of China (Hebei) Pilot Free Trade Zone (for Trial Implementation)”, which puts forward 40 supportive measures in terms of innovating incentive and supportive methods, administrative and regulatory systems, trade facilitation measures, and industrial development modes, to emancipate the mindset, boldly innovate, and endeavor to make Zhengding Area a new high ground for reform and opening up in the new era of reform and opening up the new high ground. The Opinions make it clear: support qualified medical institutions in Zhengding Area to carry out stem cell-related research work, and give 5 million yuan of scientific research funding support to the clinical research projects on stem cell clinical frontier medical technology that have been completed for the record.
Support qualified medical institutions in Zhengding Area to cooperate with registered biomedical enterprises in Zhengding Area to establish demonstration centers and public technology platforms for the application of gene testing technology and declare them to the National Development and Reform Commission in accordance with the procedures, and give 5 million yuan of scientific research funding support for the construction projects formally approved.
20 Nanjing, Jiangsu Province: FTZ to carry out research and application of cell therapy technology
On December 18, 2019, the Nanjing Municipal Party Committee and Municipal Government held a press conference on China (Jiangsu) Pilot Free Trade Zone Nanjing Area, which is worth paying attention to, this press conference was hosted by Zhang Jinghua, Standing Committee of the Jiangsu Provincial Committee and Secretary of the Nanjing Municipal Party Committee, and was released by Han Liming, Deputy Secretary of the Nanjing Municipal Party Committee and Acting Mayor of Nanjing Municipal Party Committee, who supported the development of the Nanjing FTZ, “1+9 The “1+9” documents were released at the same time.
The day's conference also released the “CPC Nanjing Municipal Committee Nanjing Municipal People's Government on Promoting the High-Quality Development of the Nanjing Area of China (Jiangsu) Pilot Free Trade Zone”, as well as investment and trade facilitation, industrial capital guidance, internationalization of education and health, human resources development, innovation and ecology, planning and natural resources elements to ensure that the life and health industry, the integrated circuit industry, and the development of financial innovation, and other 9 Specialized support measures. The Opinions are divided into six major parts, with a total of 28 articles, 11 of which involve the first of their kind in the country.
The document clearly proposes to promote stem cell clinical research and cell therapy and other cutting-edge medical technology research and application.
In the Measures on Supporting the Internationalization of Education and Health Care in Nanjing Area of China (Jiangsu) Pilot Free Trade Zone, it is proposed that qualified institutions designated in the area should declare to carry out research and application of cutting-edge medical technologies such as stem cell therapy, cell therapy, cellular immunotherapy and gene therapy.
21 Yunnan CPPCC: Focus on supporting the development of stem cell industry
On July 3, 2019, the Yunnan Provincial Committee of the Chinese People's Political Consultative Conference (CPPCC) held a key proposal, “Proposal on Key Support for the Development of the Stem Cell Industry as a Pilot Industry for ‘Healthy Life Destination’ in Our Province”, to vigorously develop the stem cell industry from “stem cell application” to “medical research”. “medical research”, and then ‘recreation’ whole industry chain of ‘big health industry’.
On November 14, 2019, the People's Government of Yunnan Province officially released “Several Opinions on Accelerating the High-Quality Development of Biomedical Industry” on its official website. The opinion is clear: actively develop cell therapy products focusing on stem cells. Strengthen the applied basic research and translational research on cell products, and promote the filing of clinical research projects on cell products. Relying on scientific research institutions or relevant enterprises in Yunnan, build cell product preparation centers.
Promote the construction of cell product testing centers and actively carry out regional third-party testing and evaluation services. Develop a unified, standardized and regulated technical quality system, and promote the commercialization and application of clinical research results. Encourage enterprises to accelerate the development of immune cell therapy products around malignant tumor treatment and abnormal immune function.
As governments at all levels follow the guidelines of national policies, they continue to introduce policy rules to support the development of the stem cell industry in order to promote the original breakthroughs and transformation of stem cells, encourage and support the development of basic and clinical research on stem cells, and encourage the improvement of China's regenerative medicine field through the innovative power of enterprises and other organizations.
Stem cell technology has incomparable advantages over traditional treatment. With the gradual improvement of stem cell technology, gradual expansion of infrastructure, gradual upgrading of industrial chain, and gradual increase of policy support, the stem cell industry will surely become a pillar industry of biomedicine for the benefit of the country and the people, and the industry as a source of the clear stream of caring for the Huaxia nation will surely dedicate their strength.
At the beginning of 2020, in the fight against the new crown pneumonia epidemic, stem cell technology has become a treatment option with good clinical prospects for the treatment of new crown pneumonia, and its safety and effectiveness have been verified by clinical and scientific research data, which has provided strong scientific and technological support for overcoming the epidemic.
When General Secretary Xi inspected the scientific research on the prevention and control of C.pneumonia in Beijing on March 2, he emphasized the need to strengthen basic research in the field of life sciences and breakthroughs in key core technologies in healthcare, and to accelerate the improvement of strategic scientific and technological strength and strategic reserve capacity in the field of epidemic prevention and control and public health, which pointed out the specific direction for promoting scientific research on the prevention and control of the epidemic and the great development of the biomedical industry.






